Botox Report - Why animal testing for cosmetics is banned and mice still die for botox
- News
On 24 April 2025, ECEAE member Tierschutz Austria published a comprehensive report on animal testing for botulinum toxin products, better known as Botox.

Despite the EU’s animal testing ban for cosmetics, tens of thousands of mice are still used annually to test Botox products - even when these are used solely for aesthetic purposes. This is possible because injected products like Botox are classified as medicinal products under EU law and therefore exempt from the cosmetics testing ban.
LD50 Testing: Outdated, Inhumane, and Still Legal
Many manufacturers still use the LD50 (lethal dose) test for each batch of Botox, in which mice are injected with varying doses of the neurotoxin to determine the concentration that kills 50% of the animals. This causes severe suffering: affected animals die from paralysis, respiratory failure, or dehydration. Although non-animal methods - such as cell-based assays and organ-on-a-chip systems - are available and validated, they are not yet legally required, and uptake remains inconsistent.
Ireland: The EU Hotspot for Botox Animal Testing
Our analysis of official data and unpublished project documentation reveals that Ireland is currently the central hub for Botox-related animal testing within the EU. In 2022, approximately 75% of all regulatory animal tests conducted in Ireland were for Botox - a total of around 36,000 mice. By 2024, over 100,000 mice were authorized for Botox testing in Ireland, with the trend continuing upward.
This dramatic rise is largely hidden from public view due to a critical transparency gap in EU and national reporting systems.
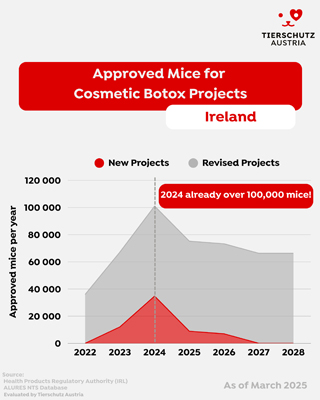
The Transparency Gap: Project Extensions Keep the Public in the Dark
EU law requires non-technical summaries (NTS) of new animal testing projects to be published in the ALURES database. However, this requirement does not apply to project renewals or extensions - a loophole that is being heavily exploited in Ireland.
Instead of submitting new applications, Irish authorities frequently approve extensions to existing Botox testing projects, thereby authorizing tens of thousands of additional animals without triggering any new public reporting. In 2022, no new Botox projects were published, yet our research - supported by the Irish Anti-Vivisection Society - revealed that three existing projects were extended, covering tens of thousands of mice for LD50 testing.
These extensions are invisible in the EU’s public database, meaning:
- There is no EU public record of the animal numbers approved via extensions or renewals,
- No opportunity for external scrutiny or debate, and
- No scientific accountability regarding 3R implementation (Replacement, Reduction, Refinement).
This practice severely undermines the transparency goals of Directive 2010/63/EU and calls into question whether the EU’s system is fit for purpose when it comes to high-volume, high-severity procedures like LD50 testing for Botox.
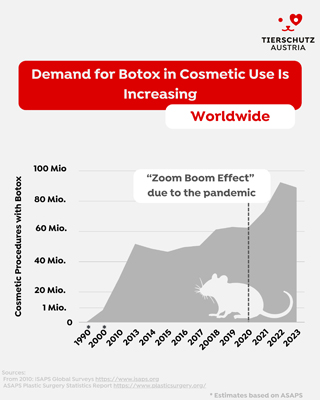
Increasing Demand, Rising Animal Use
Globally, aesthetic Botox procedures have surged - from fewer than 1 million in 2000 to nearly 90 million in 2023. The “Zoom Boom-Effect” during the COVID-19 pandemic further accelerated demand. In Europe, the market is projected to grow nearly 5% annually until 2030. This boom drives continued reliance on animal-based testing - despite the existence of non-animal methods.
Methodology
This report is based on a combined analysis of publicly available data from EU and national animal testing databases, as well as project summaries accessed with the support of the Irish Anti-Vivisection Society and literature research.
Key sources included:
- ALURES Statistical EU Database: to assess overall numbers of animal procedures in EU27 + Norway.
- ALURES Non-Technical Summary Database (NTS): to identify new Botox-related testing projects. Search terms included "botulinum" and related keywords. Texts in national languages were machine-translated for comparative analysis
- Unpublished extended project approvals in Ireland (2019–2024): obtained through NGO collaboration and used to quantify the additional number of mice approved via project extensions.

Further information
- Botox Report by Tierschutz Austria (German) as PDF
- Petition by Tierschutz Austria against Botox testing (in German)
