SCIENTIFIC ARGUMENTS AGAINST ANIMAL EXPERIMENTS
Respect for the lives of animals is the main reason many people reject animal experiments. But even if one focuses on humans with regard to the ethical justifiability of animal experiments, it is the wrong approach. There is no ethical dilemma “animal suffering instead of human suffering”, but rather abundant scientific evidence that animal experiments harm humans rather than benefitting them.
Humans are not 75 kg rats
Every human being is an individual. That is why it is very common for a pharmaceutical drug to benefit one person and not another. This statement even applies to identical twins. Age, gender, background or environment also play a large part in how one reacts to certain substances. It is not without reason that there are specialist areas in medicine, such as andrology, gynaecology, gender medicine, geriatrics and paediatrics; or statements such as “women are sick differently than men”, “a child is not a small adult”. (1,2,3,4) Nowadays there is much talk of "individualised medicine", but if humans are not comparable amongst themselves, how should animal results be transferred to humans?

Humans and animals – as well as the individual animal species amongst – can differ significantly from each other, for instance in terms of anatomy, organ function, metabolism and nutrition. At the same time, we share more than 96% of our genes with chimpanzees (5, 6) and at least 85% with mice. (7,8) However, even if our genome largely matches that of apes or even mice, this is not a mathematical equation in the sense that sharing common genes equates to being identical. Human and animal genetic material is partly subject to completely different regulation. During the course of evolution, there have been drastic changes and adjustments in this regard. Which genes are activated or not, or how they influence each other is very different between humans and animals. (9) Therefore, the approximately four percent difference between the genetic material of humans and apes (chimpanzees) means that the latter cannot contract hepatitis B, malaria or AIDS. (10,11) There are also major differences between humans and non-human primates with regard to the development and structure of their brains. The development of the human brain takes about three and a half times as long as that of apes. This is not surprising, considering that the final size of a human brain is about four times that of chimpanzees or gorillas. The human brain has areas not found in apes: for language, reading, singing, poetry, mathematics, sports, abstract thinking. The number of nerve connections and the surface of the brain are also many times greater in humans. Despite these considerable differences, apes are still used in brain research as a “model” for humans. (12) In Germany in 2017, about 70% of the animals used were mice and about 11% were rats. According to one study, only 43% of the side effects in humans can be predicted using these species (13), so tossing a coin would be more accurate. Rats and mice correspond to only 60% with regard to the carcinogenic or embryo-damaging effects of substances. (13) A study by American scientists compared the immune system responses of humans and mice to blunt force trauma, burns or sepsis. They discovered significant differences, with humans responding to inflammation much more strongly and sometimes for up to half a year, whereas the immune system’s response in mice already wears off after a few days. (14) This is not surprising, considering that mice, unlike humans, can thrive on spoiled foods. The dose of bacteria sufficient to cause lethal sepsis in humans is one million times less than the lethal dose in mice. (15,16) Therefore, it is no surprise that none of the 150 substances that have been proven to be effective when treating severe inflammation in animals has been successful in humans. (17,18,19,20) In a Swiss study, researchers had to concede that – despite decades of successful research on rodents – there are still no therapies that can repair damaged human spinal cords. The reason named for the poor transferability of test results is the considerable anatomical differences between rats and humans. (21) A British team of researchers examined the results of animal experiments that had been conducted in order to assess the risk of deformities in unborn children. It turned out that nearly half of the substances known to cause deformities in humans had previously been classified as harmless in animal studies. Conversely, nearly half of the drugs that women can take during pregnancy without problems were also considered unsafe in animal studies. (22) Here too, the comparison with tossing a coin is very apt. It would not have delivered any worse results; but it would have avoided many cruel animal experiments.
Artificially induced animal symptoms are meant to simulate human diseases
Many diseases that occur in humans do not occur or are rare in animals. For example, animals do not get Alzheimer’s or Parkinson’s diseases. However, in order to be able to research them, the animals are genetically, surgically, medically or behaviourally manipulated so that they develop symptoms similar to those of the disease. Common “animal models” for cancer are, for example, “generated” by genetic manipulation or the injection of human cancer cells into mice. A tremor reminiscent of Parkinson's disease is also displayed by monkeys, mice, or rats who have a specific neurotoxin injected into the brain. In depression research, rats are placed in a water tank from which they cannot escape. If they stop swimming, they are considered to be depressive. (23)

Many animal experiments are conducted only to develop such animal models. Subsequently, drugs or other forms of therapy are tested on these animal models. If the symptom disappears, it is assumed that a remedy has been found for the disease in humans. The researcher learns nothing about the human disease itself and crucial aspects of the disease in humans are ignored, for the artificially induced symptoms have nothing in common with the actual human disease they are meant to simulate. Whether a person becomes ill depends on many factors: genetics, nutrition, stress, environment. Also, an illness usually comprises multiple symptoms. These are individually reproduced in different animal models. One animal model commonly used for Alzheimer’s are transgenic “Alzheimer’s mice” (mice into whose genome human genes were introduced). Although the genetic manipulation leads to the animals developing deposits (plaques) in the brain similar to those typical for Alzheimer’s in humans, or have impaired memory, a complete Alzheimer’s dementia with all the symptoms displayed by humans cannot be reproduced. Genetics only plays a part in 3% of cases of Alzheimer’s disease. In addition to a genetic predisposition, there are other internal and external influences (such as age, previous severe head injuries, unhealthy lifestyle) that contribute to whether or not the disease breaks out in humans. (24, 25) However, these factors cannot be taken into account in research on mice. So far, more than 300 therapeutic approaches have been successfully tested on “Alzheimer's mice” and other animals. Yet despite decades of research, this has not produced a single drug that can cure or halt the disease in humans. (26) Multiple sclerosis (MS) is simulated in mice in a variety of ways. A study conducted by the Hannover School of Veterinary Medicine showed why, after about 100 years of animal experimental MS research, the causes of this human disease are still largely unknown. The study analysed publications on three common animal models in which MS is simulated in different ways (excessive immune response to the body’s own nerve cells – triggered by injecting a protein, central nervous system virus infection and genetic manipulation). According to the study, animal experiments cannot simulate human disease at the level of individual genes. Only 12 of the nearly 5,000 genes responsible for MS could also be detected in animals. These behaved even opposite to the human genes. All twelve genes were down-regulated in MS patients but up-regulated in animal models (27); This demonstrates once more that biology is not mathematics and that matching genes in no way implies transferability. The fact that healing artificially induced symptoms in animals does not lead to the desired success in humans is most apparent in cancer research. Quotes such as “The history of cancer research has been a history of curing cancer in the mouse. We have cured mice of cancer for decades—and it simply didn’t work in humans” (Richard Klausner, M.D., former director of the US National Cancer Institute) (28) and “We have learned well how to treat cancer in mice and rats but we still can’t cure people”(29) bear witness to this. Even after more than 200 years of cancer research on animals, many kinds of cancer in humans still cannot be cured, with the annual number of deaths from cancer continuously rising.
Poor reproducibility is countered by standardisation
In order to render results reproducible, i.e. repeatable, animal experiments are carried out under so-called standardised conditions. Age, sex and weight of the animals should therefore be as equal as possible. In addition, feeding (type and quantity of feed) and housing of the animals (cage size, temperature, light and ventilation conditions) are identical. That this has little in common with the complex reality is beyond question. And yet one and the same animal experiment, even under these “laboratory conditions”, usually leads to very different results. This is partly because the animals are not machines, but individuals who can react in completely different ways. In addition, the stress to which the animals are exposed greatly influences the experimental outcomes. Simply handling the animals has a considerable influence on the results, because mere touching already causes severe stress symptoms. (30) Even the gender of the animal experimenter is important. According to one study, male researchers exert greater stress on mice and rats than their female colleagues, influencing the results more strongly. (31) Standardisation completely ignores the fact that the genesis and development of human disease depends on the variability of internal and external influences. What person lives, eats and behaves “under laboratory conditions”?
Animal experiments do not bring patient safety, but are more like a lottery
Due to the aforementioned differences between humans and animals as well as the artificially induced symptoms, it is not surprising that in spite of intensive animal experiments during the last 150 years two thirds of all diseases are still not curable in humans, nor are their causes known. (32) According to a review, none of 119 cures promised during the last 30 years has been fulfilled. (33) No other scientific method is as unreliable and unpredictable as animal experiments. What animals – and which species – react exactly the same way to a substance as humans only becomes apparent after testing on humans. Animal testing has never been validated, but has still been the “gold standard” in biomedical science for decades. That applies nowhere else, so why is it accepted in the important area of human health? After being tested on animals, new therapies need to be tested on humans in clinical trials to determine their safety and efficacy. Due to the poor transferability of the animal-based results to humans, however, this step represents an incalculable and thus highly unethical risk. This is now recognised by more and more researchers. (34) Between 92.5 and 95% of all pharmaceutical drugs found to be effective and safe in animal studies fail in the subsequent clinical phases 1 to 3 in humans. This is because they either do not work or show severe side effects. (35,36,37) Drug scandals such as TGN1412 (England, 2006) and Bia 10-2474 (France, 2016) highlight the enormous risk of animal testing. In the two examples, the substances proved to be safe and effective in the so-called preclinical phase (in animal experiments). In both cases, even non-human primates, our closest relatives, were given 500-fold and 650-fold doses compared to phase 1 of the clinical trials with humans. (38, 39) This first stage, testing substances on healthy volunteers, proved a disaster. In 2006, the use of a potential drug for multiple sclerosis resulted in multiple organ failure in six human subjects; in 2016, the administration of a potential drug for treating chronic pain not only caused severe neurological damage to five subjects but also led to one death. (40,41) The fact that in both cases only a relatively small number of persons were harmed is due to the fact that such trials are conducted with extreme caution – an indication that scientists and pharmaceutical industry themselves do not trust animal experiments. Even if a drug is approved, that does not mean it is safe for humans, because about one third of the 5 – 7.5% of the drugs that are approved are later withdrawn from sale or have warnings issued due to serious side effects. (42) According to a study by the Hannover Medical School, 58,000 deaths each year in Germany can be attributed to inappropriate medication and undesired side effects of drugs. This figure only includes those patients who die in internal medicine departments in hospitals, and does not even include patients from other departments, outpatients or people who died at home. Also not included are patients with chronic long-term consequences of drug side effects. According to the author of the study, Prof. Frölich, the study only documents “a small part” of the malady. (43,44) In 2010, another study documented 25,000 deaths per year from side effects and drug interactions. However, it acknowledges that it is difficult to determine exact numbers. (45) If you take a look at a pharmaceutical drug’s package insert, it is hardly surprising that only a few lines describing the desired effects are by far outnumbered by the warnings regarding undesired side effects. Since 2013, there is even a black triangle warning patients about new drugs that have not yet been fully tested. Such a drug is then “under additional monitoring”. (46) There are many past or present examples of drugs being withdrawn from the market. (47) The painkiller VIOXX had to be withdrawn in 2004 after 5 years because of the increased incidence of heart attacks and strokes. (48) It is said to have caused 88,000 to 140,000 cases of severe heart disease in the United States alone. (49,50) Zinbryta, touted as a “miracle cure for multiple sclerosis”, was withdrawn from the market in March 2018 after several patients had meningitis and liver failure. (51)
Important drugs not discovered because of animal experiments?
Thus, there are many substances that are effective and safe in animals and ineffective in humans and/or cause strong side effects. But what does that mean conversely with regard to substances that were eliminated in animal experiments but would have been an important therapeutic option for humans? Penicillin, aspirin and paracetamol are examples of medicines that were developed around 100 years ago, are still commonly used in human medicine today and are usually well tolerated by the patient. If these substances had been tested for their effectiveness and safety with today's routine animal experiments, they would never have been available on the market. Although even pregnant women and their unborn children can tolerate aspirin well, it causes deformities in the offspring of many species (including mice, rats and monkeys). Penicillin is lethal to guinea pigs and rabbits, paracetamol causes cancer in rodents and is toxic to cats. Cyclosporine A has been used successfully in humans for years to prevent graft rejection, and then only because results from tests on humans were so promising that the poor results of animal experiments were ignored and the drug was brought onto the market nonetheless. (52) Such examples could go on indefinitely. It can therefore be summarised that animal experiments do not identify risks relevant to humans, but equally important is the fact that animal-based research assumes risks to humans that do not actually exist, resulting in important drugs not being approved or used. Testing on animals is thus not only unethical toward animals, but also toward (terminally) ill people hoping for a cure.
Animal experiments’ alleged success stories
It is often suggested that animal testing has been crucial for biomedical innovation and success. However, simply because animal experiments were conducted in the context of some discoveries, this does not automatically lead to the conclusion that they were only achieved by virtue of the animal experiments. In most cases, non-animal methods such as clinical observations lead to medical breakthroughs, and it is only with hindsight that these observations are “reproduced” in animal experiments. If one then comes to a similar result, the animal experiment is made responsible for the success. (53)
Animal experiments in basic research
In recent years, animal experiments in so-called basic research has increased continuously. In Germany in 1991, they amounted to 13%, whereas today almost 60% of animal experiments are conducted in basic research. (54) One main reason for this increase is genetic engineering. Basic research focuses on the curiosity-driven acquisition of knowledge. By definition, the primary goal is not a specific benefit, e.g. discovering a drug or therapy. Because of this, no limits are set to researchers’ ingenuity and there are many examples of frivolous experiments. Freedom of research is even protected by the German Constitution, regularly taking precedence over animal welfare, likewise enshrined in the Constitution. (55) Heart attacks or strokes lead to cerebral hypoxia, a life-threatening lack of oxygen in the brain. Researchers had the idea that investigating a species adapted to a low-oxygen environment could point the way to a possible therapy for hypoxia. Therefore, experiments were performed on naked mole-rats, a specialised rodent species found in the soil of East Africa. Scientists investigated how long they can survive with very little or no oxygen and carried out control experiments with mice. At 0% oxygen, the naked mole-rat survived 18 minutes at a normal body temperature of 30 °C. At an elevated body temperature of 37 °C, they asphyxiated after 6 minutes. The naked mole-rats did not survive 30 minutes without oxygen. However, by comparison, mice suffocated after only 45 seconds. (56)
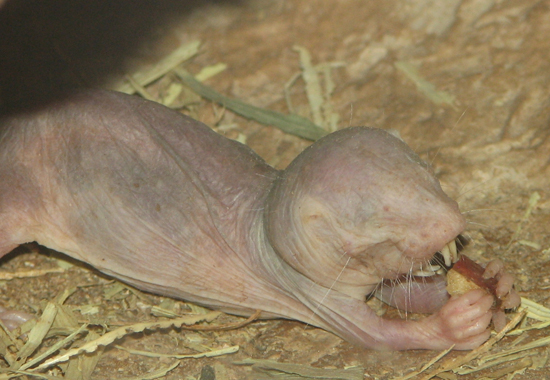
How does this extremely cruelly acquired knowledge help us? In the course of evolution (researchers speak of 30 million years), naked mole-rats have adapted to their low-oxygen environment by adjusting their metabolism to break down fructose. Incidentally, whales use a different way of adapting to phases with little oxygen when diving: they can store oxygen in the muscle protein myoglobin. (57) In depression research, drugs are tested on flies, which – after repeatedly being subjected to vibration stress in a dark tube over a period of days and then having their wings clipped off – lose interest in feeding. (58) In the course of evolution, all animals – as well as human beings – have developed divergently over millions of years, specialising in their respective habitats. Spiders can build webs to which they themselves do not adhere, fireflies glow in the dark, lizards regrow their tails; these are all examples of the wonderful specialisations that exist in the animal kingdom. How is knowing how naked mole-rats can deal with oxygen deficiency relevant to humans suffering a stroke or heart attack? We will never spontaneously adapt our metabolism in an emergency. Or what does a fly stressed by constant vibration and amputated wings have to do with depressive humans? Faith may move mountains, but we will never find a drug that replaces 30 million years of evolution in our favour. No wonder, then, that reviews regarding the relevance of the results of this basic research for humans are devastating. Lindl et al. examined 51 animal experiments from the years 1991-1993 with regard to their relevance to humans 10 years on. They found that only 0.3% of the studies they examined showed a direct correlation between animal experimental findings and the results found in humans. But even in those the hypotheses confirmed by animal experiments could not be implemented in clinical therapies for humans. Either no therapeutic effect was detectable or the findings in humans even contradicted the results of the animal experiments. (59) For lack of a “better” study, the German Primate Center in Göttingen even used the review article by Lindl et al. on its website until 2016 to justify basic research with monkeys. Thus, a 99.7% error rate was considered acceptable for the lottery that animal experiments are. A follow-up study published in 2011 by Lindl et al. dealt with the clinical relevance of the same animal experiments 17 years on. Once more, there was no indication of a direct correlation between animal experiments and treatment methods. (60) Another study analysed more than 25,000 publications from leading basic research journals between 1979 and 1983. 101 articles contained statements that the results had great potential for clinical use, but only 5 of them resulted in limited clinical applications by 2003, and only one case was followed by widespread clinical use (i.e. a new drug). In other words, the “success rate” of the publications examined was only a negligible 0.024% or even 0.004%. (61)
Why are animal experiments still conducted?
It is truly astonishing that a method that is both outdated and demonstrably unsuccessful has lasted so long. In a free-market economy, success rates well below one percent, or results that could even be surpassed by tossing a coin, would normally be fatal. Not so in the “gold standard” that animal experiments represent; for that tradition goes back a century and a half and is deeply rooted in the minds of most researchers and thus also in their laboratories. Young scientists – assuming they do not question the system - automatically “grow up” with animal experiments and take them for granted. (62) If you want to make a career as a scientist, you have to publish your results. A journal’s importance is rated using so-called impact factors. The more papers you publish in journals with a high impact factor, the higher you can climb the career ladder. The quality of a researcher is thus not measured by how many people were helped by his or her work, but rather the number of articles published. (63) However, journals with a particularly high impact factor mainly publish animal studies. (64,65) The vicious cycle continues, because the number of publications determines funding allocations. Billions are spent on funding for animal experiments, but animal-free research must make do with single-digit millions; the current distribution of funding is 99.x% for animal experiments and 0.y% for non-animal research. (65, 66, 67) Career and earning opportunities are therefore predominantly in the field of animal research, while researchers who decide to pursue a career in in vitro research must have a lot of perseverance in facing up to an establishment that clings to animal experiments as its “gold standard”. There are other reasons for continuing animal experiments. Animal experiments function as an “alibi” for the pharmaceutical industry. If corporations stick to legally required testing of their products on animals, they cannot easily be held liable if the product turns out to be harmful to humans. (62,68) Many institutions and companies also benefit from animal testing. Laboratories are rebuilt and refurbished. Such items of expenditure are usually in the double-digit million range. (69) A breeding industry “produces” animals “tailored” to the experimental projects. As a researcher, you can even order artificially harmed animals in a catalogue, simply compiling the symptoms the animals should have. As if that were not macabre enough, these breeding companies even make special offers. The price of a single mouse can range from about €80 to €80,000 for specifically genetically modified mice. (70,71,72) In 2016, one million “normal” and one million genetically modified mice were used and killed in experiments in Germany. Assuming an estimated average of €80 for “normal” mice or €2,000 as the average price for genetically modified mice, the cost of mice alone is € 2.08 billion a year. This may be a rough estimate, but in light of a complete lack of transparency on the part of the responsible authorities it is a viable way of bringing some light into the darkness. Animals that do not have the desired gene alteration and therefore die or are killed, and animals kept “in stock”, are not even included in the above figures. (73) We are talking about a gigantic, multi-billion dollar market dependent on continued animal testing.
The exit
Discussing whether or not animal testing made sense decades ago does not bring humanity any further and serves only as justification. We live in the 21st century with wonderful opportunities and should use them consistently and sustainably! There are innovative methods available that focus on humans and their individual diseases, and do not have to take the fallacious detour via laboratory animals. Population studies and patient studies, autopsies and cell cultures are only the beginning. In the age of computer software with “artificial intelligence”, imaging and micro-fine measurement capabilities, it is completely unacceptable to keep clinging to an outdated and irrelevant method. Personalised research is the key. Skin cells taken from a patient can be used to produce various specialised organ cells via so-called induced pluripotent stem cells (Nobel Prize 2012), which develop into organoids and are researched either singly or with other organs on multi-organ chips. By administering a drug to such organoids or multi-organs-chips, it is then possible to investigate how it will work in the patient in question. Thus, it is already possible to conduct research on the model of a (sick) person. It may be only a model, but it is a relevant one! Although mice and rats are living organisms, they are foreign species and therefore irrelevant to humans! (74.75)

Conclusion
Medical progress is important, but animal experiments are the wrong path! Not only does it lead to false results, but it even impedes medical progress. This was true in the past decades, but it is even more so in light of today’s high-tech research methods focusing on humans.
9 August 2018 Dr. Gaby Neumann V.D.M.
References
(1) Heinrich, C: Bin ich anders krank als du? Die Zeit, 23.5.2017 >>
(2) Universitätsklinikum Heidelberg: Kinder sind keine kleinen Erwachsenen. Press release 15.05.2002 (PDF)
(3) Verband der forschenden Pharmaunternehmen, Broschüre „Zur Sache“ 2006, Nr. 8: Arzneimittel für Kinder (PDF)
(4) Warum viele Asiaten keinen Alkohol vertragen. Die Welt online, 20.01.2010
(5) National Institutes of Health, Pressemitteilung von 2005: New Genome Comparison Finds Chimps, Humans Very Similar at the DNA Level >>
(6) Varki, A und Nelson, DL: Genomic Comparisons of Humans and Chimpanzees. Annual Review Anthropology 2007: 36; 191-209 (PDF)
(7) National Human Genome Research Institute, Pressemitteilung vom 23.07.2010: Why Mouse Matters >>
(8) Church, DM et al.: Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biology 2009: 7 (5); e 1000112 >>
(9) Yue, F et al.: The mouse ENCODE Consortium. An Integrated and Comparative Encyclopedia of DNA Elements in the Mouse Genome. Nature 2014: 515; 355-364
(10) Greek, R et al.: A Scientific Case for the Elimination of Chimpanzees in Research. Project Release & Restitution for Chimpanzees in U.S. Laboratories 2005 (PDF)
(11) Bodderas, E: Was Affen besser können als wir. Die Welt, Wissen, 23.04.2007 >>
(12) Ménache, A: The replacement of non-human primates in brain research. One Voice 2010 >>
(13) Olson, H et al.: Concordance of the Toxicity of Pharmaceuticals in Humans and in Animals. Regulatory Toxicology and Pharmacology 2000: 32; 56–67. 20000122
(14) Seok, J et al.: Genomic responses in mouse models poorly mimic human inflammatory diseases. PNAS 2013: 110 (9); 3507–3512
(15) Sauter, C und Wolfensbergeret, C: Interferon in human serum after injection of endotoxin. The Lancet 1980: 2(8199); 852–853
(16) Råberg, L et al.: Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science 2007: 318 (5851); 812–814
(17) Hotchkiss, RS und Opal, S: Immunotherapy for sepsis—a new approach against an ancient foe. New England Journal of Medicine 2010: 363(1); 87–89
(18) Hotchkiss, RS et al.: The sepsis seesaw: Tilting toward immunosuppression. Nature Medicine 2009: 15 (5); 496–497
(19) Wiersinga, WJ: Current insights in sepsis: From pathogenesis to new treatment targets. Current Opinion in Critical Care 2011: 17 (5); 480–486
(20) Mitka, M: Drug for severe sepsis is withdrawn from market, fails to reduce mortality. JAMA 2011: 306 (22); 2439–2440
(21) Friedli, L et al.: Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Science Translational Medicine 2015: 7 (302); 302ra134
(22) Bailey, J et al.: The future of teratology research is in vitro. Biogenic Amines 2005: 19 (2), 97-145
(23) Ärzte gegen Tierversuche: Datenbank Tierversuche.de
(24) Deutsche Alzheimer Gesellschaft, Informationsblatt 4 von Dezember 2016: Die Genetik der Alzheimer-Krankheit (PDF)
(25) Bundesministerium für Familie, Senioren, Frauen und Jugend, Wegweiser Demenz: Ursachen der Alzheimer-Krankheit >> (retrieved 31.7.2018)
(26) Langley, GR: Considering a new paradigm for Alzheimer´s disease research. Drug Discovery Today 2014: 19 (8); 1114-1124
(27) Raddatz, BB et al.: Transcriptomic Meta-Analysis of Multiple Sclerosis and Its Experimental Models. PLOS ONE 2014: 9; e86643
(28) Cimons, M et al.: Cancer Drugs Face Long Road From Mice to Men. Los Angeles Times, 06.05.1998 >>
(29) Safer Medicine, Quotes from Doctors & Researchers, Cancer 2007, Prof. Colin Garner >>
(30) Balcombe, JP et al.: Laboratory routines cause animal stress. Contemporary Topics in Laboratory Animal Science 2004: 43 (6); 42–51
(31) Sorge, RE et al.: Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nature Methods 2014: 11; 629–632 >>
(32) Ruppert, T: Wie entsteht ein neues Medikament. Verband forschender Pharma-Unternehmen, Presentation, 19.11.2010 (PDF)
(33) Gericke, C: Heilsversprechen der tierexperimentellen Forschung. Ärzte gegen Tierversuche e.V. 2017 >>
(34) Arzneimittelentwicklung – Rationaler, aber nicht vollends rational. Studium.at, der Online-Tutor, 11.10.2017 >>
(35) Hay, M et al.: Clinical development success rates for investigational drugs. Nature Biotechnology 2014: 32 (1); 40-51
(36) Arrowsmith, J: A decade of change. Nature Reviews Drug Discovery 2012: 11; 17-18
(37) KMR Group, Press Release vom 8.8.2012: Annual R&D General Metrics Study Highlights New Success Rate and Cycle Time Data (PDF)
(38) Attarwala, H: TGN1412: From Discovery to Disaster. Journal of Young Pharmacists 2010: 2(3); 332-336
(39) Rapport du Comité Scientifique Spécialisé Temporaire (CSST) „Inhibiteurs de la FAAH (Fatty Acid Amide Hydrolase)" sur les causes de l’accident survenu à Rennes lors d’un essai clinique de Phase 1 en janvier 2016; 18.04.2016 >>
(40) Wikipedia-Eintrag zu „TGN1412“ >> (retrieved 31.07.2018)
(41) Wikipedia-Eintrag zu „BIA 10-2474“ >> (retrieved 31.07.2018)
(42) Downing, NS et al.: Postmarket safety events among novel therapeutics approved by US Food and Drug Administration between 2001 and 2010. JAMA 2017: 317(18); 1854-186
(43) Schnurrer, JU und Frölich, JC: Zur Häufigkeit und Vermeidbarkeit von tödlichen unerwünschten Arzneimittelwirkungen. Internist 2003: 44; 889-895
(44) Wikipedia-Eintrag zu „Jürgen C. Frölich“ >> (retrieved 31.07.2018)
(45) Bis zu 25.000 Todesfälle durch Medikamente. Süddeutsche Zeitung, 17.05.2010 >>
(46) Bundesinstitut für Arzneimittel und Medizinprodukte (2013): Arzneimittel unter zusätzlicher Überwachung >>
(47) Nörenberg, E und Gericke , C: Liste von Risikomedikamenten. Ärzte gegen Tierversuche 2016 >>
(48) Kowitz, D: Zu Risiken und Nebenwirkungen. Stern, 14.10.2007 >>
(49) Brumfiel, G: Painkiller blamed for heart-disease epidemic. Nature News, 25.01.2005 >>
(50) Vioxx wohl für bis zu 140.000 Herzinfarkte verantwortlich. FAZ, 25.01.2005 >>
(51) Daclizumab (Zinbryta), Arznei-News, 07.03.2018 >>
(52) Cohen, DJ et al.: Cyclosporine: A New Immunosuppressive Agent for Organ Transplantation. Annals of Internal Medicine 1984: 101 (5); 667–682
(53) Feuerlein, K: Errungenschaften der Medizin ohne Tierversuche. Ärzte gegen Tierversuche 2018 >>
(54) Bundesministerium für Ernährung und Landwirtschaft (2018): Verwendung von Versuchstieren im Jahr 2016 >>
(55) Grundgesetz für die Bundesrepublik Deutschland, 13.07.2017
(56) Park, TJ et al.: Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017: 356; 307-311
(57) Patalo, F: Die Evolution wiederholt sich doch. Spiegel online, 18.04.2015 >>
(58) Ries, AS et al.: Serotonin modulates a depression-like state in Drosophila responsive to lithium treatment. Nature Communications 2017: DOI:10.1038/ncomms15738
(59) Lindl, T et al.: Tierversuche in der biomedizinischen Forschung - Eine Bestandsaufnahme der klinischen Relevanz von genehmigten Tierversuchsvorhaben: Nach 10 Jahren keine Umsetzung in der Humanmedizin nachweisbar. ALTEX 2005: 22(3); 143-151
(60) Lindl, T und Völkl, M: No clinical relevance of approved animal experiments after seventeen years. ALTEX 2011: 28; 242-243
(61) Chalmers, I et al.: Research: increasing value, reducing waste 1: How to increase value and reduce waste when research priorities are set. The Lancet 2014: 383 (9912); 156–165
(62) Die Ökonomie der Tierversuche. Handelsblatt, 04.02.2018 >>
(63) Wikipedia-Eintrag „Impact Factor“ >> (retrieved 31.07.2018)
(64) Loll, A und Schmidt, T: Charité Berlin, Umdenken in der medizinischen Forschung. Deutschlandfunkkultur, 19.10.20117 >>
(65) Loll, A: Tierversuche – Geboren, um sinnlos zu sterben. Zeit online, 24.4.2016 >>
(66) Ärzte gegen Tierversuche, Pressemitteilung vom 24. Januar 2018: Skandalös: Milliarden Steuergelder fließen in Tierversuche, nur geringe Beträge in tierversuchsfreie Forschung >>
(67) Loll, A: Wir tun einfach nicht genug für Alternativen. Frankfurter Allgemeine vom 02.11.2017 >>
(68) Greek, R und Swingle Greek, J: Sacred Cows and Golden Geese: The Human Cost of Experiments on Animals. Bloomsbury Academic, New York, 2000
(69) Bürgerschaft der Freien und Hansestadt Hamburg, Haushaltsplan 2017/2018, Einzelplan 3.2 Behörde für Wissenschaft, Forschung und Gleichstellung, Nachbewilligung nach § 35 Landeshaushaltsordnung (LHO), Neubau und Ertüchtigung der Forschungstierhaltung der Medizinischen Fakultät der Universität Hamburg/Universitätsklinikum Hamburg-Eppendorf (UKE)
(70) Tolba, R, Institut für Versuchstierkunde der RWTH Aachen. WDR 5 Panel discussion, 25.10.2012
(71) Testbiotech: „Stoppt Investionen in Tierleid“, 2015 (PDF)
(72) Mice play a critical role in medical research. NBC News, 03.06.2006 >>
(73) Kleine Anfrage Ursula Hammann (Bündnis 90/Die Grünen) 2014 zu „Zucht- und Vorratshaltung von Tieren in Tierversuchslaboren in Hessen“ an das Ministerium für Umwelt, Energie, Landwirtschaft und Verbraucherschutz (Drucksache 18/7733)
(74) Strittmatter, S und Gericke, C: „Woran soll man denn sonst testen?“, Ärzte gegen Tierversuche, 2018 (PDF)
(75) Edington, CD et al.: Interconnected Microphysiological Systems for Quantitative Biology and Pharmcology Studies. Scientific Reports 2018: DOI:10.1038/s41598-018-22749-0
